Pertumbuhan Mikroorganisme
Pertumbuhan pada mikroorganisme uniseluler, seperti bakteri, khamir atau protozoa, adalah peningkatan jumlah populasi.
Hal tersebut menggambarkan peningkatan biomassa. Biomassa adalah jumlah total materi seluler pada suatu sistem.
Estimasi Jumlah Mikroba
Pengukuran jumlah bakteri (bisa juga untuk mikroorganisme uniseluler lainnya), ada 2 kategori:
- Menghitung jumlah total sel
- Menghitung sel-sel hidup saja
Microscopic Counts
Total cell counts
Secara umum dilakukan dengan pengamatan mikroskopik. Menggunakan kaca mikroskop khusus yang menandai suatu area tertentu.
Sel-sel yang tampak pada area tersebut menggambarkan jumlah sel per unit volume.
The method may be made more accurate by the use of a fluorescent dye such as acridine orange, which binds to DNA, and hence avoids confusion with non-cellular debris.
However, such methods cannot differentiate between living and non-living cells.
Their usefulness is further limited by the fact that the smallest bacteria are difficult to resolve as individual cells by light microscopy.
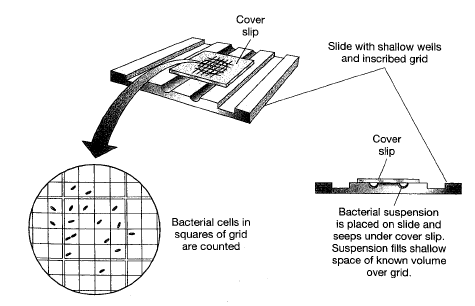
Estimation of total cell numbers by direct microscopic measurement.
Metode lain untuk menghitung sel pada sampel cair dengan menggunakan flow cytometer.
Flow cytometer merupakan mesin yang menggunakan sinar laser dan perangkat alat elektronik untuk menghitung sel-sel individu.
Flow cytometer banyak digunakan dalam menghitung dan membedakan sampel darah untuk sampel klinik.
Dalam ekologi mikrobiologi juga digunakan untuk membedakan tipe-tipe sel untuk tujuan isolasi.
Kelemahan:
- Tanpa pewarnaan, sel-sel mati tidak dapat dibedakan dengan sel hidup
- Sel-sel yang kecil sulit diamati di bawah mikroskop
- Ketepatan sulit diperoleh
- Memerlukan mikroskop fase kontras jika sampel tidak diwarnai
- Sel-sel yang motil harus di immobilisasi sebelum dihitung
- Debris pada sampel dapat di salah artikan sebagai sel-sel mikroba
Viable Counts
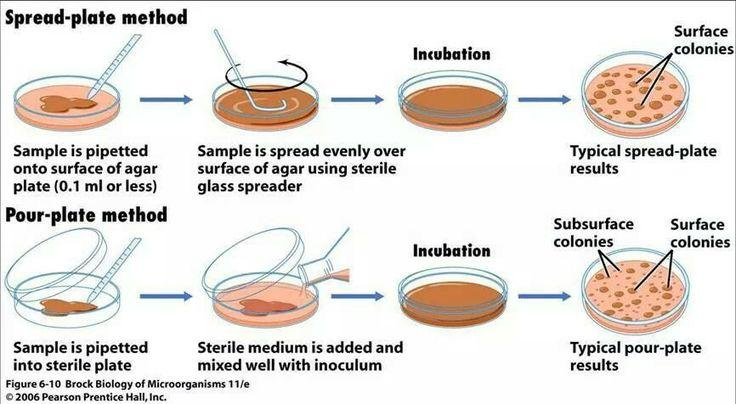
A viable cell count, on the other hand, is a measure of the number of living cells in a sample, or more specifically those capable of multiplying and producing a visible colony of cells.
It is most commonly estimated by spreading a known volume of cell suspension onto an agar plate, and counting the number of colonies that arise after a period of incubation.
The viable count is also called plate count → we determine the number of cells in a sample
capable of forming colonies on a suitable agar medium.
The assumption made in the viable counting procedure is that each viable cell can grow and divide to yield one colony. Thus, colony numbers are reflection of cell numbers.
Ada 2 cara untuk melakukan plate count:
- Spread-plate method
- Pour plate method
Spread-plate Method
Pada spread-plate method, volume (biasanya 0,1 ml) kultur yang telah diencerkan disebar di atas permukaan agar cawan menggunakan spreader.
Cawan kemudian diinkubasi hingga terjadi pertumbuhan koloni. Total koloni yang tumbuh dihitung.
Pour-plate Method
- Sejumlah volume kultur (0,1—1 ml) di pipet ke dalam cawan Petri steril.
- Medium agar yang masih cair kemudian dituang ke dalam cawan tersebut.
- Agar dan kultur di mixed hingga rata.
- Cawan kemudian diinkubasi hingga tumbuh koloni.
- Koloni yang tumbuh tidak hanya di atas permukaan agar tetapi juga bisa di dalam agar.
Pengenceran Suspensi Sel:
- Dalam viable counts, pengukuran sel yang valid adalah sekitar 30—300 koloni.
- Jika jumlah koloni terlalu kecil, signifikansi statistiknya terlalu rendah.
- Jika jumlah sel terlalu banyak, beberapa koloni bisa saja bergabung sehingga terjadi kesalahan penghitungan.
- Untuk menghindari kesalahan-kesalahan tersebut, sampel sebaiknya diencerkan.
Kesalahan dalam Plate Counting:
- Inkonsistensi plating (pemipetan yang tidak akurat)
- Kurang teraduk secara rata
Turbidimetric Methods
Turbidimetric methods measure the change in optical density or absorbance of the medium, that is, how much a beam of light is scattered by the suspended particulate matter.
They can be carried out very quickly by placing a sample in a spectrophotometer.
Values of optical density can be directly related to bacterial numbers or mass by reference to a standard calibration curve.
Thus, an estimate of bacterial numbers, albeit a fairly approximate one, can be obtained almost instantaneously during an experimental procedure.
Other indirect methods of measuring cell density include wet and dry weight estimations, and the measurement of cell components such as total nitrogen, protein or nucleic acid.
THE KINETICS OF MICROBIAL GROWTH
- Unicellular organisms divide by binary fission; each cell grows to full size, replicates its genetic material then divides into two identical daughter cells.
- By identical means, two cells divide into four, four into eight and so on, leading to an exponential increase in cell numbers : 1→2→4→8→2ⁿ
- If we were to plot the number of cells in a population against time, we would get an exponential curve.
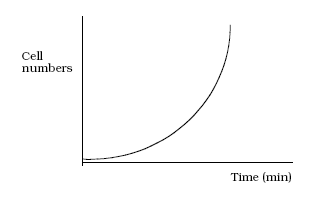
- It is more convenient when plotting a growth curve to plot the logarithm of cell numbers of against time, giving us a straight line.

- Such exponential growth cannot continue indefinitely, however, and growth usually slows down due to either the supply of nutrients becoming exhausted, or because metabolism leads to an accumulation of harmful waste substances.
- Unicellular growth usually occurs in a series of different phases.

A microbial growth curve. The four phases of a typical growth curve are shown.
1. Lag phase
When an inoculum of bacteria is first introduced into some growth medium, it will probably require a period to adapt to its new surroundings – the less familiar these are, the longer the period of adaptation.
If, for example, the carbon source in the medium is unfamiliar, the cells will need time to synthesise the necessary enzymes for its metabolism.
The length of the lag phase will also depend on the age and general health of the cells in the inoculum.
During this period, there is no net increase in bacterial numbers, however the cells are metabolically active.
2. Log (exponential) phase.
When the bacteria have acclimatised to their new environment and synthesised the enzymes needed to utilise the available substrates, they are able to start regular division by binary fission.
This leads to the exponential increase in numbers referred to above.
Under optimal conditions, the population of cells will double in a constant and predictable length of time, known as the generation (doubling) time.
The value for the widely used laboratory bacterium E. Coli is 20 min, and for most organisms it is less than an hour.
There are some bacteria, however, whose generation time is many hours. Thus, during exponential growth, the number of cells can be expressed as: N𝚻 = N₀ x 2ⁿ
3. Stationary phase
As discussed above, the exponential phase is limited by environmental factors, and as the rate of growth slows down, the culture enters the next phase.
The leveling out of the growth curve does not mean that cell division has ceased completely,
But rather that the increase due to newly formed cells is cancelled out by a similar number of cell
deaths.
Eventually, however, as the death rate increases, the overall numbers fall and we enter the final phase of growth. Death phase.
As cells die off and the culture is unable to replace them, the total population of viable cells falls. This is the death (or decline) phase.
Batch Culture and Continious Culture
The phases of growth described above apply to a batch culture.
In this form of culture, appropriate nutrients and other conditions are provided for growth, then an inoculum is added and the culture incubated.
No further nutrients are added and no waste products are removed, thus conditions in the culture are continually changing.
This results in active growth being of limited duration for the reasons outlined above.
Sometimes it is desirable to keep the culture in the logarithmic phase, for example if the cells are being used to produce alcohol or antibiotics.
In a continuous culture, nutrient concentrations and other conditions are held constant, and the cells are held in a state of exponential growth.
This is achieved by continuously adding fresh culture medium and removing equal volumes of the old.
Parameters such as pH can also be monitored and adjusted. The equipment used to do this is called a chemostat.
It produces a steady-state culture whose population size is kept constant by careful control of flow rates and nutrient concentrations.

Continuous culture of microorganisms: the chemostat.
*Sumber: Nitya Wita Utama
Baca Juga :